From:
The Infections Department of the SHARY ZEDEK Medical, Israel.
AGAVI Ltd., Jerusalem, Israel
Corresponding Author:
OSNAT AVIGAIL ANAVI PHRMD
Anti-bacterial viruses Performance of Air Filtration System AGAVI
Introduction
As part of the growing concern of hospital- acquired infections, in August 2019, AGAVI LTD Air performed a trial in collaboration with The Infections Department of The SHARY ZEDEK Medical Center in the installation of AGAVI filter for the purpose of disinfecting and purifying the air from various pollutants.
Initial results showed a remarkable ability of the purification filter to disinfect various bacteria. With the outbreak of the COVID-19 pandemic, in Wuhan, China, focus of the global medical community has changed from preventing hospital acquired bacterial infections into preventing viral spread of SARS-CoV-2.
Previous experimental results obtained in collaboration with AGAVI LTD Air demonstrate the ability to filter a series of high-risk pathogens including various viruses, such as Influenza H1N1 and Influenza H5N1. We were able to obtain a Coronavirus and build a model to test filter for their ability to filter or eliminate the virus. In May 2019, the company began a clinical experiment to test the effectiveness of AGAVI filter disinfection capabilities on the Coronavirus. The aim of the trial was to quantify the properties of the AGAVI filter to purify air contaminated by a Coronavirus similar in size with the SARS-Cove- 2.
Methods
AGAVI Air Disinfection Technology
We putted Silver coated filters installed in four different types of rooms, in Share Zedek Medical Center: a regular three-patient room in internal medicine, an isolation room with negative pressure and hepa-filter ventilation system, an isolation room with positive pressure and hepa-filter ventilation system and an operating room with 12 air exchanges/hour. The medical activity in these rooms continued as usual during the study period.
The first stage of the study focused on change in bacterial and fungal pathogens in these rooms. In the test rooms we putted certified quantitative air sampling instrument placed on specific position for the whole trial. Positions chosen to achieve symmetry in the room with respect to walls, ventilation inlets/outlets etc.
Air samples collected before the installation of Silver coated filters, two weeks and four weeks after the implementation. These air samples cultured for bacterial (on Tryptic soy 5% sheep blood agar plates) and fungal (on Sabouraud dextrose agar plates) pathogens. After the sampling all plates incubated for 2 days at 37 °C (± 2.5).). After the incubation all visible colonies counted. The results reported as colony forming units (CFU)/m3.
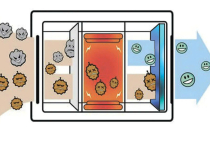
OUR PRODUCT FOR AIR CONDITIONING SYSTEM IS FILTER ION SILVER AND HEPA ION SILVER
HEPA ION SILVER filter
HEPA stands for high-efficiency particulate air, and it is an efficiency standard for air filters. The efficiency is measured in the ability of the filter to retain particles larger than 0.3 µm. These filters are used in environments that require contamination control such as food and pharmaceutical industries, hospitals, and semiconductors.
The ION SILVER WE ARE ADD TO DESTROY THE VIRUSES structure of those filters consists of randomly arranged fibers, typically from polyethylene. The diameter of the fiber ranges between 0.5-2 µm. The efficiency of the filter is determined by the fiber diameter, filter thickness, and the face velocity (which is the air speed in the inlet of the filter).
Coronavirus detection
Virus propagation
Virus propagation was performed in embryonated chicken eggs. The allantoic fluid was harvested 48 h post-inoculation (PI) and stored at −80 ◦C, until used for RNA extraction.
Virus Detection: Real-time RT-PCR assay
A conserved region of 336 b located at nucleotide position 741–1077 of the H120 strain N gene sequence (GenBank accession no. AM260960) was used to design primers and probe for the real-time RT-PCR assay. Amplification plots were recorded, analyzed, and the threshold cycle (Ct) determined with the Step One software, version 2 (Applied Biosystems).
1. Test Description:
Tested materials and pretesting preparation:
Elements of the filtration system were tested separately to define antiviral properties of each component.
HEPA filter was cut to squares of 0.5 X 0.5 cm.
ION SILVER Fabric has been turned into four-layer squares measuring 0.5 x 0.5 cm.
Performance: The tests were done at The Share Zedek Laboratory, Testing process:
HEPA filters and ION SILVER has been contaminated by wetting of viral suspension (10 µl per each piece) and exposed for 10 min. Within exposure, we extracted virus residuals from the samples by rinsing of the samples with 0.1 ml of dPBS. aterials and equipment:
a) PCR machine: Step One pus RT-PCR System, A&B applied Bio-Systems.

Virus detection: Presence of the virus in exposed suspension was checked by RT-PCR technique using relevant reagents.
2. Testing Results:
Results processing: The data measured by the RT-PCR system at # 32 was used to evaluate the results of the experiment. This have been defined as the last point in which measurements of control samples do not show saturation of detector and prevent misunderstanding of the results.
Results:
| Sample | Corona virus Reduction Ratio [%] |
|---|---|
| AGAVI filter | 99.998 |
| HEPA rep1 | 29.7243 |
| SCF rep.1 | 69.9744 |
| Sterionizer ™ LP rep.1 | 59.9651 |
| Sterionizer ™ HP rep.1 | 69.9429 |
| UVC LED rep.1 | 59.9631 |
Conclusions:
Each of the tested components of the AGAVI Air Device was able to significantly reduce the viral load as measured by PCR. In addition, an air measuring device was taken, a device collected air and threw on a plate, the plate was tested in the laboratory and a decrease in the number of bacteria,
it was proven that the colonies of the bacteria decreased from 104 to 18 -less 85%, and colonies of yeast decreased from 16 to 9- less 56%.
This promising result is the basis of more studying the performance of the AGAVI Air device in closed spaces.